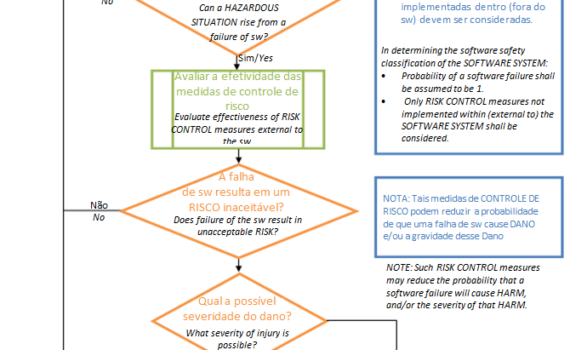
IEC 62304 is an international harmonized standard that provides guidance to the manufacturer on the planning, development and post-market surveillance activities for medical device software to ensure that companies comply with the requirements of international regulatory agencies. Its first version was published in 2006 […]

